 The recent publication of the first in vivo CAR-T clinical study for SLE is a major milestone (full study: https://www.nejm.org/doi/full/10.1056/NEJMc2509522). This new targeted LNP platform offers huge potential— patient accessibility, scalability, speed, and cost-efficiency—but how does it measure up to ex vivo approaches?
The recent publication of the first in vivo CAR-T clinical study for SLE is a major milestone (full study: https://www.nejm.org/doi/full/10.1056/NEJMc2509522). This new targeted LNP platform offers huge potential— patient accessibility, scalability, speed, and cost-efficiency—but how does it measure up to ex vivo approaches?
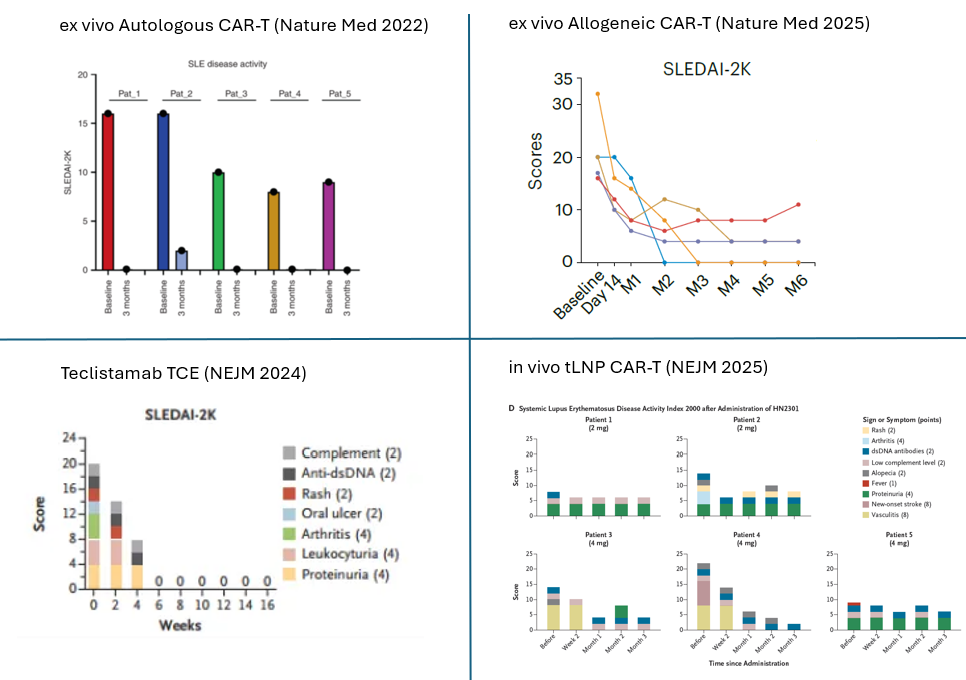
Let’s look qualitatively at SLEDAI-2K score reductions across four modalities:
🔹 Autologous CAR-T
🔹 Allogeneic CAR-T
🔹 TCE antibodies
🔹 The new tLNP therapy
While ex vivo CAR-T continues to show the most robust and consistent clinical effect, the in vivo tLNP approach is rapidly closing the gap.
In vivo CAR-T is still in its infancy, and its success hinges on delivery specificity, efficiency and lasting expression. That’s where Tiva Biosciences https://tiva.bio comes in. We’re enabling the next breakthrough by giving researchers fast, reliable access to the tLNP platform they need to assess and refine their molecules.
Ready to transform ex vivo therapies to in vivo using tLNP technology? let’s connect and move in vivo CGT forward, faster.
#in vivo CAR-T #CGT #tLNP
https://www.nejm.org/doi/full/10.1056/NEJMc2509522
https://www.nejm.org/doi/full/10.1056/NEJMc2407150
https://www.nature.com/articles/s41591-022-02017-5
https://www.nature.com/articles/s41591-025-03899-x

